Guanidinium/ammonium competition and proton transfer in the interaction of the amino acid arginine with the tetracarboxylic 18-crown-6 ionophore†
Abstract
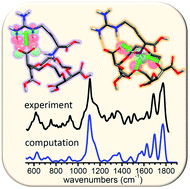
The recognition of arginine plays a central role in modern proteomics and genomics. Arginine is unique among natural amino acids due to the high basicity of its guanidinium side chain, which sustains specific interactions and proton exchange biochemical processes. The search for suitable macrocyclic ionophores constitutes a promising route towards the development of arginine receptors. This study evaluates the conformational features involved in the binding of free arginine by the polyether macrocycle (18-crown-6)-tetracarboxylic acid. Infrared action vibrational spectroscopy and quantum-chemical computations are combined to characterize the complexes with net charges +1 and +2. The spectrum of the +1 complex can be explained in terms of a configuration predominantly stabilized by a robust bidentate coordination of guanidinium with a carboxylate group formed from the deprotonation of one side group of the crown ether. The released proton is transferred to the amino terminus of arginine, which then coordinates with the crown ether ring. In an alternative type of conformation, partly consistent with experiment, the amino terminus is neutral and the guanidinium group inserts into the crown ether cavity. In the +2 complexes, arginine is always doubly protonated and the most stable conformations are characterized by a tripodal coordination of the ammonium –NH3+ group of arginine with the oxygen atoms of the macrocycle ring, while the interactions of the amino acid with the side carboxylic acid groups of the crown ether acquire a remarkable lesser role.


 Please wait while we load your content...
Please wait while we load your content...