Oxocarbon-functionalized graphene as a lithium-ion battery cathode: a first-principles investigation†
Abstract
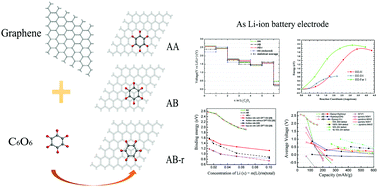
In recent years, organic-based, especially carbonyl-based, Li-ion battery electrode materials have attracted great attention due to their low-cost, environmentally friendly nature and strong Li-ion bonding abilities. However, new research is required to further increase the electron mobility and cycling performance of organic materials. The performance of a high-carbonyl C6O6 molecule-functionalized graphene electrode for Li-ion batteries is investigated using the density functional theory. The binding energy calculations indicate that the C6O6 molecule is adsorbed on graphene via physisorption. C6O6@graphene maintains excellent electronic conductivity with 1 to 6 Li ions. By our statistical method, the reduced voltage of the C6O6@graphene cathode displays a voltage between 2.6 V and 1.5 V with 2 phases from 1 to 6 Li ions with energy density of approximately 155 mA h g−1. The results obtained reveal that C6O6@graphene is a promising electrode material for renewable Li-ion batteries.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...