Concurrent presence of on- and off-pathway folding intermediates of apoflavodoxin at physiological ionic strength†
Abstract
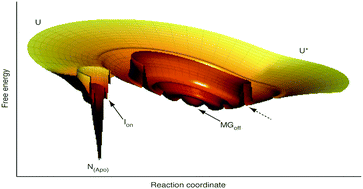
Flavodoxins have a protein topology that can be traced back to the universal ancestor of the three kingdoms of life. Proteins with this type of architecture tend to temporarily misfold during unassisted folding to their native state and form intermediates. Several of these intermediate species are molten globules (MGs), which are characterized by a substantial amount of secondary structure, yet without the tertiary side-chain packing of natively folded proteins. An off-pathway MG is formed at physiological ionic strength in the case of the F44Y variant of Azotobacter vinelandii apoflavodoxin (i.e., flavodoxin without flavin mononucleotide (FMN)). Here, we show that at this condition actually two folding species of this apoprotein co-exist at equilibrium. These species were detected by using a combination of FMN fluorescence quenching upon cofactor binding to the apoprotein and of polarized time-resolved tryptophan fluorescence spectroscopy. Besides the off-pathway MG, we observe the simultaneous presence of an on-pathway folding intermediate, which is native-like. Presence of concurrent intermediates at physiological ionic strength enables future exploration of how aspects of the cellular environment, like for example involvement of chaperones, affect these species.


 Please wait while we load your content...
Please wait while we load your content...