Conformational switching via an intramolecular H-bond modulates the fluorescence lifetime in a novel coumarin–imidazole conjugate†
Abstract
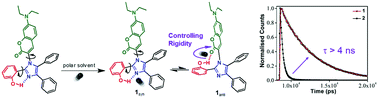
Achieving synthetic control over light-driven molecular dynamics is essential for designing complex molecule-based devices. Here we design a novel coumarin–imidazole conjugate (1) whose excited state structural dynamics are primarily controlled by a distant intramolecular H-bonding interaction within the backbone. The coumarin conjugate is based on a 1,2,4,5-aryl substituted imidazole framework (aryl = –Ph and –PhOH) covalently connected to the coumarin moiety via a C–N bond. A carefully positioned OH group in the aryl part of the imidazole fragment resulted in achieving two dissimilar O–H⋯N and O–H⋯O distal intramolecular hydrogen bonding interactions. NMR studies in conjunction with density functional theory (DFT) at the B3LYP/6-311G(d,p) level of theory show the existence of two ground state conformers with a rotational barrier of 6.12 kcal mol−1. Due to the presence of conformational isomers of 1, the local excited state dynamics of the parent coumarin get biased towards a long-lived fluorescence state with diminished non-radiative decay channels. Time-resolved emission studies show an ∼4–5 times increase in the excited state lifetime in 1 when compared to coumarin–imidazole conjugates, 2 and 3, without the OH group. Solvent dependent studies show that solvent polarity, the H-bond donating ability and viscosity dictate the conformational distribution in the ground state and the dynamical evolution to the final emissive state. Our studies highlight the importance of rotamerism around the C1–C4 single bond, which leads to rigidification along the coumarin–imidazole backbone through a combination of distal H-bonding and solvent interactions. The concept of new emission signaling pathways caused by conformational switching between two states offers a new paradigm to introduce functional allostery in macromolecular backbones.


 Please wait while we load your content...
Please wait while we load your content...