High-pressure and temperature dependence of the spontaneous resolution of 1,1′-binaphthyl enantiomers†
Abstract
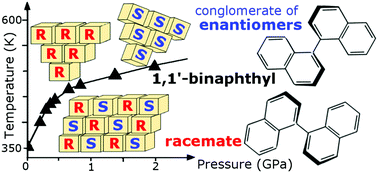
High pressure increases the temperature of the spontaneous resolution of 1,1′-binaphthyl conformational enantiomers in the crystalline state, which confirms that the enantiomers and racemates are stabilized in the molecular environments in compressed structures. The established pressure–temperature (p–T) preference diagram for the racemate–enantiomer spontaneous crystallization corresponds to a boundary between solid phases, as it is consistent with the Clausius–Clapeyron equation, however, the hysteresis of such a solid-state transformation extends to very high pressure, to 3 GPa, at least according to this study. High-pressure X-ray diffraction study on single crystals of 1,1′-binaphthyl racemate and enantiomer reveals their monotonic compression and structural changes up to 3 GPa. It also reveals the increasing role of intermolecular interactions for stabilizing the structures, despite the exceptionally large density difference between the racemate (1.277 g cm−1) and enantiomers (1.183 g cm−1).


 Please wait while we load your content...
Please wait while we load your content...