Investigation of the interaction of amyloid β peptide (11–42) oligomers with a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) membrane using molecular dynamics simulation†
Abstract
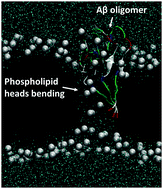
Some amyloid related proteins/peptides are involved in aggregation and pore formation in phospholipid membranes (cell membranes), which result in a variety of neurological disorders such as Alzheimer's disease, Parkinson's disease and Huntington disease. In this research, the mechanism of pore formation by β amyloid (Aβ) peptides was investigated using molecular dynamics simulation by simulating the interaction of the Aβ(11–42) peptide, with a lipid membrane and the potential of the mean force of interaction was evaluated. A 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) membrane system with different cholesterol concentrations was used to simulate the neural cell membrane. The results indicated that Aβ(11–42) peptide oligomers with peptide numbers larger than two were more likely to lead to lipid deformation and water channels, and the free energy of penetration into the membrane decreased with the increasing number of peptides. Increasing the concentration of cholesterol leads to a higher energy barrier for the penetration of peptide into the lipid bilayer thereby protecting the membrane. The results of this research have potential application in the prevention of pore formation by Aβ aggregates on the lipid membrane.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...