Electron and excitation energy transfers in covalently linked donor–acceptor dyads: mechanisms and dynamics revealed using quantum chemistry†
Abstract
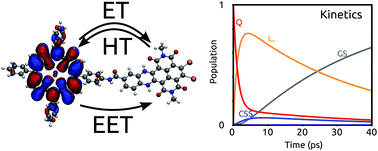
Photoinduced electron transfer (ET), hole transfer (HT), charge recombination (CR) and energy transfer (EET) are fundamental mechanisms, which occur in both natural and artificial light harvesting systems. Here, we present a computational strategy which determines ET, HT, CR and EET rates in a consistent way and merges them in a kinetic model to reproduce the net excited state dynamics. The effects of the solvent are included in all steps of the calculations making the present strategy a useful tool for a rational design of charge and energy transfer processes in complex systems. An application to covalently linked zinc and free-base porphyrin–naphthalenediimide dyads is presented. For each of the two systems, ultrafast optical spectroscopy experiments have shown a specific photophysics with different processes taking place simultaneously. The model reveals that such a diversity is mainly due to the different relative stability of the charge-separated state, while the electronic couplings for charge and energy transfer processes are quite similar in the two dyads.


 Please wait while we load your content...
Please wait while we load your content...