Discrimination between hydrogen bonding and protonation in the spectra of a surface-enhanced Raman sensor†
Abstract
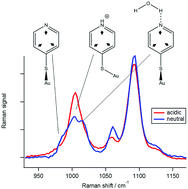
We investigate the surface-enhanced Raman spectra of 4-mercaptopyridine on gold in a variety of acids. 4-Mercaptopyridine is a known pH sensor which exhibits characteristic spectral changes when the pH is changed. Here we show with the help of experiment and density functional calculations that the ring breathing mode is also highly sensitive to hydrogen bonding. Its spectral signature is a broad band with up to three contributions from free, protonated and hydrogen-bonded 4-mercaptopyridine. Unlike pyridine in solution, where protonation leads to a higher ring breathing frequency than hydrogen-bonding, we find that protonated adsorbed 4-mercaptopyridine possesses a frequency which is lower than the corresponding hydrogen-bonded species. The Raman spectra indicate an orientation change of the aromatic ring in acidic solutions, which could be caused by a cation/π interaction between protonated and deprotonated 4-mercaptopyridine. As the frequencies of the three species are well separated, adsorbed 4-mercaptopyridine can probe more complex changes in the solution environment than just pH.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...