CoO microspheres and metallic Co evolved from hexagonal α-Co(OH)2 plates in a hydrothermal process for lithium storage and magnetic applications†
Abstract
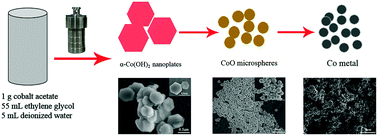
CoO microspheres and metallic Co could be successfully synthesized by simply reacting cobalt acetate with a mixture solvent of ethylene glycol and deionized water in a hydrothermal process for different times. As the reaction proceeded, α-Co(OH)2, CoO and metallic Co were produced. To understand the phase evolution processes from α-Co(OH)2 to CoO and then metallic Co, a range of time-dependent experiments were carried out, and the intermediate products obtained at different reaction times were investigated in detail. The investigation revealed that CoO microspheres were actually evolved from α-Co(OH)2 as a precursor. Just elongating the reaction time, CoO microspheres could be further reduced to metallic Co. With a pure ethylene glycol medium for the same reaction, only α-Co(OH)2 could be generated, indicating an important role of water. When the obtained CoO microspheres were used as anode materials for lithium-ion batteries, they delivered a specific capacity of 803 mA h g−1 at 0.1 A g−1 with a retention of 453 mA h g−1 after 70 cycles. Meanwhile, the magnetic properties of the obtained CoO microspheres and metallic Co were investigated, with the CoO microspheres showing an antiferromagnetic behavior and the metallic Co exhibiting ferromagnetic characteristics. This study suggested a novel method for synthesizing CoO with a uniform microsphere morphology and bulk metallic Co easily.


 Please wait while we load your content...
Please wait while we load your content...