Radiation chemistry of solid acetone in the interstellar medium – a new dimension to an old problem
Abstract
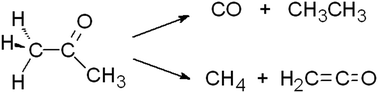
A laboratory investigation of acetone, an interstellar and cometary molecule, has produced new results concerning its decomposition in a radiation environment. Mid-infrared spectroscopy has been used to follow amorphous acetone's destruction by ionizing radiation (1 MeV protons) at 20 K. Radiation products identified are the CH4, CO, and CO2 usually made in such experiments, along with ketene, allene, and the acetonyl radical, all identified here for the first time in irradiated solid acetone. Evidence for the reduction product 2-propanol was suggestive, but a firm identification could not be made either for it or for the C2 hydrocarbons (i.e., C2H6, C2H4, C2H2). The acetyl radical was not observed as a radiation product. Isotopically labeled reagents were used to demonstrate ketene formation and to emphasize that multiple approaches are needed for robust assignments of infrared spectral features of irradiated icy solids. Results from a supporting radiation experiment with isotopically labeled acetic acid are described. Comparisons are made to a previous study of acetone's stability in extraterrestrial radiation environments, and caution is urged in measuring and interpreting CO abundances in irradiated icy solids.
- This article is part of the themed collections: 2018 PCCP HOT Articles and Theory, experiment, and simulations in laboratory astrochemistry


 Please wait while we load your content...
Please wait while we load your content...