Computational study on the luminescence quantum yields of terbium complexes with 2,2′-bipyridine derivative ligands†
Abstract
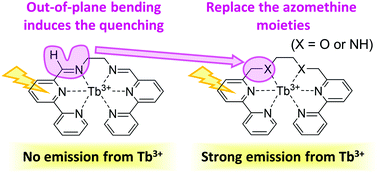
Terbium complexes are widely used as luminescent materials because of their bright green emission and sharp emission spectra and the independence of their emission wavelengths from the surrounding environment. The luminescence quantum yield (LQY), however, heavily depends on the surroundings, and an appropriate ligand design is indispensable. In this study, we focus on a Tb3+ complex coordinated by a 2,2′-bipyridine derivative ligand (L1), whose LQY is almost zero at room temperature [M. Hasegawa et al., New. J. Chem. 2014, 38, 1225] and compare it with a Tb3+ complex with a bipyridine ligand, which is widely used as a photo-antenna ligand. To discuss the LQYs of the complexes, we computed their energy profiles, i.e. the energetic and structural changes during the emission and quenching processes. The low LQY of the TbL1(NO3)2 complex was explained by the stability of the minimum energy crossing point between the potential energy surfaces of the ligand-centered lowest triplet state and the ground state, which was induced by the out-of-plane bending of the azomethine moiety. The most efficient way to improve the LQY by modification of the ligand is to replace the azomethine moieties by other functional groups, such as ether or reduced azomethine groups, whose minimum energy crossing points are unstable enough to reduce the rate of the quenching processes.
- This article is part of the themed collection: Complex molecular systems: supramolecules, biomolecules and interfaces


 Please wait while we load your content...
Please wait while we load your content...