A “turn-off” red-emitting fluorophore for nanomolar detection of heparin†
Abstract
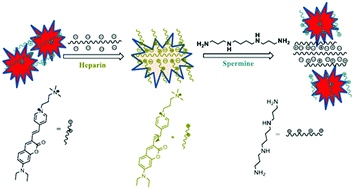
A simple fluorophore bearing a diethylaminocoumarin donor and a pyridinium acceptor was synthesized and utilized for the ultra-sensitive detection of heparin. The synthesized dicationic push–pull coumarin derivative emits strongly in the red-region (665 nm) and detects nanomolar concentrations (14.8 nM to 148 nM) of heparin in HEPES buffer and FBS serum solutions. The dication exhibits excellent fluorescence selectivity and sensitivity towards heparin over its analogues such as chondroitin 4-sulfate (CS), hyaluronic acid (HA) and dextran. This fluorescence assay is a convenient, sensitive method for monitoring heparin levels in biological samples. These findings were confirmed using coarse-grained Monte Carlo simulations, which provide us with a rationale for the selective binding of heparin.


 Please wait while we load your content...
Please wait while we load your content...