Structures and infrared spectra of calcium phosphate clusters by ab initio methods with implicit solvation models
Abstract
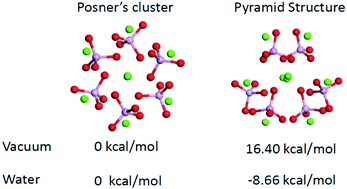
Since the first detection of pre-nucleation clusters during the formation of calcium phosphate minerals, determining such clusters’ compositions and structures has become crucial for understanding the early-stage nucleation of these minerals in solutions. In previous experimental studies, the composition and sizes of pre-nucleation clusters have been calculated, but their structural information has been difficult to determine because they are very small (<1 nm). In this study, we examined the structures and infrared spectra of small- and medium-sized calcium phosphate clusters using ab initio calculations combined with implicit solvation models. Adding solvent effects increased the possibility of the existence of alternative configurations of calcium phosphate clusters other than their compact configurations. The calcium atoms had a tendency to be located outside of the clusters to coordinate with water molecules in the aqueous environment. The computed infrared spectra of extended small calcium phosphate clusters captured some of the features measured in the in situ infrared spectra, which supports the network structures proposed by large-scale molecular dynamics studies and X-ray adsorption near-edge spectra. The relative stabilities of medium-sized Ca9(PO4)6 clusters with respect to the stability of Posner's cluster in water were also reviewed. We found that in water, alternative structures with low symmetry or large dipole moments had lower energies than Posner's cluster.


 Please wait while we load your content...
Please wait while we load your content...