Probing the effect of different graphitic nitrogen sites on the aerobic oxidation of thiols to disulfides: a DFT study†
Abstract
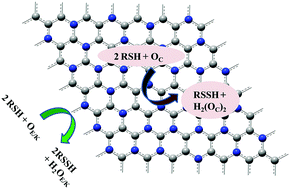
Functionalized graphene materials are well known for their application in catalyzing the aerobic oxidation of alcohols, hydrocarbons, etc. in an aqueous medium. Despite the fact that a few catalysts are known to oxidize thiols to disulfides, their selectivity is poor and requires oxidants that are not suitable in terms of the principles of green chemistry. Therefore, in this context, an attempt has been made to investigate the possibility of utilizing nitrogen doped graphene for the aerobic oxidation of thiols to disulfides using density functional theory (DFT). Our previous study (V. S. Jeyaraj, M. Kamaraj and V. Subramanian, J. Phys. Chem. C, 2015, 119, 26438–26450) has shed light on the activation of dioxygen to form activated oxygen species (AOS) at different graphitic nitrogen sites. Hence the same has been used to study the two-electron oxidation of thiophenol and methanethiol. The AOS are of three kinds: (1) peroxide type at the edges, (2) superoxide type at the center and (3) ketonic type at edges. The findings from this study indicate that the peroxide type AOS leads to selective formation of diphenyl disulfide, whereas the superoxide type at the center facilitates the formation of hydrogen peroxide which could lead to over-oxidation of disulfide. The oxidation of aromatic thiols (thiophenol) by the ketonic type of AOS is nearly a barrier-less reaction (0.67 kcal mol−1). Similarly, AOS at the edges with the peroxide form can oxidize aliphatic thiols (methanethiol) with a less barrier of 1.55 kcal mol−1, which can be a spontaneous reaction. The mechanism of oxidation is completely different from the oxidative pathway of alcohols by the same AOS. The formation of S–OH species is strictly avoided by the strong stabilization of thiyl radicals over the π-surface of graphene.


 Please wait while we load your content...
Please wait while we load your content...