Central-metal effect on intramolecular vibrational energy transfer of M(CO)5Br (M = Mn, Re) probed by two-dimensional infrared spectroscopy†
Abstract
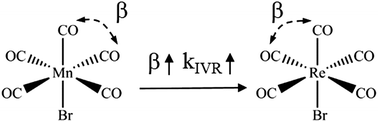
Vibrational energy transfer in transition metal complexes with flexible structures in condensed phases is of central importance to catalytical chemistry processes. In this work, two molecules with different metal atoms, M(CO)5Br (where M = Mn, Re), were used as model systems, and their axial and radial carbonyl stretching modes as infrared probes. The central-metal effect on intramolecular vibrational energy redistribution (IVR) in M(CO)5Br was investigated in polar and nonpolar solvents. The linear infrared (IR) peak splitting between carbonyl vibrations increases as the metal atom changes from Mn to Re. The waiting-time dependent two-dimensional infrared diagonal- and off-diagonal peak amplitudes reveal a faster IVR process in Re(CO)5Br than in Mn(CO)5Br. With the aid of density functional theory (DFT) calculations, the central-metal effect on IVR time linearly correlates with the vibrational coupling strength between the two involved modes. In addition, the polar solvent is found to accelerate the IVR process by affecting the anharmonic vibrational potentials of a solute vibration mode.


 Please wait while we load your content...
Please wait while we load your content...