Solid–liquid equilibria for a pyrrolidinium-based common-cation ternary ionic liquid system, and for a pyridinium-based ternary reciprocal ionic liquid system: an experimental study and a thermodynamic model
Abstract
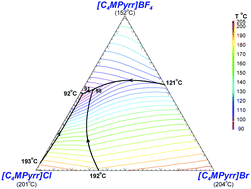
The present paper describes an experimental study and a thermodynamic model for the phase diagrams of the common-cation ternary system [C4MPyrr]Cl–[C4MPyrr]Br–[C4MPyrr]BF4 (where [C4MPyrr] refers to 1-butyl-1-methyl-pyrrolidinium) and of the ternary reciprocal system [C2Py], [C4Py]‖Cl, Br (where [CnPy] refers to 1-alkyl-pyridinium). Phase equilibria were measured by Differential Scanning Calorimetry (DSC) for two isoplethal sections in the common-cation pyrrolidinium-based ternary system. Phase diagram measurements were recently performed for the four common-ion binary subsystems and the two diagonal sections in the pyridinium-based ternary reciprocal system. In each case, the Modified Quasichemical Model was used to model the liquid solution, and the Compound Energy Formalism was used for the relevant solid solutions. For the ternary reciprocal system, the missing thermodynamic properties of the pure compounds were assessed using the Volume-based Thermodynamics (VBT) from Glasser and Jenkins, making it possible to estimate the exchange Gibbs free energy for the reaction [C2Py]Br (liquid) + [C4Py]Cl (liquid) = [C2Py]Cl (liquid) + [C4Py]Br (liquid). The experimental diagonal sections [C4Py]Br–[C2Py]Cl and [C4Py]Cl–[C2Py]Br were satisfactorily reproduced using solely the optimized model parameters for the four common-ion binary subsystems.


 Please wait while we load your content...
Please wait while we load your content...