Lithium diffusion study in Li2MnO3 and Li1.17Ni0.17Mn0.67O2: a combined experimental and computational approach†
Abstract
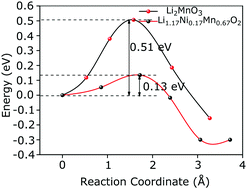
A theoretical and experimental diffusivity study of Li2MnO3 and Li1.17Ni0.17Mn0.67O2 has been carried out to investigate the effect of Mn, Ni and surrounding atoms on Li+ diffusion and to understand how the Li+ diffusion trajectory changes with different charge spheres. It is observed that due to the presence of Ni in Li1.17Ni0.17Mn0.67O2, the activation energy reduces in all the possible diffusion paths, which helps in faster Li+ diffusion. This study brings a new physical insight into Li+ diffusion based on elliptical and straight diffusion trajectories. In Li1.17Ni0.17Mn0.67O2, the Li+ diffusion mechanism in different paths based on 2b, 2c and 4h Wyckoff sites of Li has been discussed. Experimentally, the galvanostatic intermittent titration technique is adopted to identify the diffusion coefficient of Li+. The diffusion coefficient of both the compounds varies in different voltage ranges. For L2MnO3, diffusion varies from 10−11 to 10−13 cm2 s−1, whereas for Li1.17Ni0.17Mn0.67O2, diffusion varies from 10−9 to 10−11 cm2 s−1 in the voltage range of 3.7–4.7 V.


 Please wait while we load your content...
Please wait while we load your content...