Explaining the symmetry breaking observed in the endofullerenes H2@C60, HF@C60, and H2O@C60†
Abstract
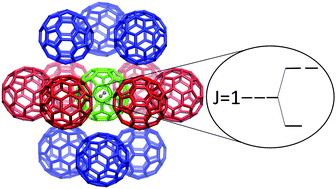
Symmetry breaking has been recently observed in the endofullerenes M@C60 (M = H2, HF, H2O), manifesting in the splittings of the three-fold degenerate ground states of the endohedral ortho-H2, ortho-H2O and the j = 1 level of HF. The nature of the interaction causing the symmetry breaking is established in this study. A fragment of the solid C60 is considered, comprised of the central C60 molecule surrounded by twelve nearest-neighbor (NN) C60 molecules. The fullerenes have either P (major) or H (minor) orientational orderings, and are assumed to be rigid with Ih symmetry. Only the central C60 is occupied by the guest molecule M, while the NN fullerenes are all empty. The key proposition of the study is that the electrostatic interactions between the charge densities on the NN C60 molecules and that on M inside the central C60 give rise to the symmetry breaking responsible for the measured level splittings. Using this model, the M@C60 level splittings of interest are calculated variationally and using perturbation theory, for both the P and H orientations. Those obtained for the dominant P orientation are in excellent agreement with the experimental results, with respect to the splitting magnitudes and patterns, for all three M@C60 systems considered, pointing strongly to the quadrupolar M–NN interactions as the main cause of the symmetry breaking. The level splittings calculated for the H orientation are about 30 times smaller than the ones in the P orientation.


 Please wait while we load your content...
Please wait while we load your content...