CO2 reduction catalysis by tunable square-planar transition-metal complexes: a theoretical investigation using nitrogen-substituted carbon nanotube models†
Abstract
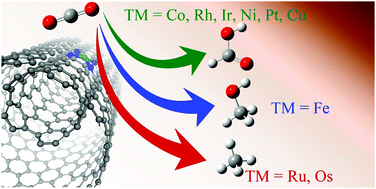
In this work, using density functional theory, we have characterized the CO2 reduction capabilities of a series of nine transition-metal-chelated nitrogen-substituted carbon nanotube models (TM-4N2v-CNT). Each of the chelated models consists of a four-N-substituted and one vacancy framework to mimic square planar homogeneous catalysts, and is coordinated to Fe, Ru, Os, Co, Rh, Ir, Ni, Pt or Cu. The results are further investigated to search for the possible electrochemical intermediates along the CO2 reduction pathway. We’ve found that all of the tested elements are predicted to favor the hydrogen evolution reaction over CO2 reduction energetically (with the exception of Cu), and that only Group 8 elements are predicted to bind CO effectively and other cases prefer HCOOH formation. The observed CO binding preference could be rationalized via ligand field theory based on the molecular orbitals of the square planar complexes. With a suitable applied voltage to stabilize all of the adsorbed CO intermediates, Ru and Os are predicted to produce CH4, whereas Fe is predicted to produce CH3OH. Increasing the curvature of the CNT could reduce the required potential in the potential-determining step substantially. However, the predicted catalytic sequence is subject to only the selection of a metal center.


 Please wait while we load your content...
Please wait while we load your content...