Bipolar charge transfer induced by water: experimental and first-principles studies†
Abstract
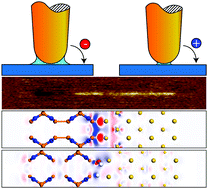
The mechanism underlying bipolar charge distribution in the context of triboelectrification remains ambiguous. Furthermore, water can either promote or inhibit triboelectrification. It was determined through experimental and first-principles calculations that water can also reverse the polarity of transferred charges and cause a bipolar charge transfer. We examined triboelectrification between an Au/Cr-coated tip and stoichiometric Si3N2 film using Kelvin probe force microscopy. In addition, we investigated the generation of a bipolar charge distribution on the insulating Si3N2 surface by controlling the frictional conditions. With regard to the effect of a water meniscus in the interface between the surface and tip, we predicted the dissociation of water molecules on the Si3N2 surface and polarity reversing induced by water via first principles. Finally, we focused on the effect of water on the dangling bonds of Si atoms and surface states of Si3N2. The results indicated that the dangling bonds and surface states are essential to a bipolar charge transfer.


 Please wait while we load your content...
Please wait while we load your content...