Melting kinetics of superheated crystals of glucose and fructose
Abstract
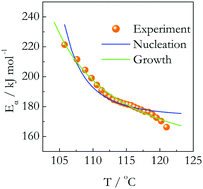
Glucose and fructose crystals undergo significant superheating during melting that allows one to study the kinetics of this process. Melting of both compounds has been studied by differential scanning calorimetry (DSC). The obtained data have been subjected to isoconversional kinetic analysis. The process has been determined to have unusually large values of the activation energy and preexponential factor that indicate that melting occurs by cooperatively breaking multiple hydrogen bonds. The experimentally determined activation energy of melting demonstrates a decrease with increasing temperature. The use of the nucleation and growth models has permitted deriving theoretical dependencies of the activation energy on temperature. Testing the theoretical dependencies against the experimental ones suggests that from either the statistical or physical viewpoint the melting kinetics should be parameterized by means of the growth model. This suggests that the mechanism of melting involves the growth of the stable melt nuclei that exist on the crystal surface below the equilibrium melting temperature.


 Please wait while we load your content...
Please wait while we load your content...