Polysulfide intercalation in bilayer-structured graphitic C3N4: a first-principles study†
Abstract
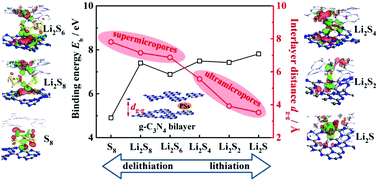
Lithium–sulfur (Li–S) batteries have attracted increasing attention due to their high theoretical capacity, being a promising candidate for portable electronics, electric vehicles and large-scale energy storage. The interactions of bilayer structured graphitic C3N4 (bi-C3N4) with S8, lithium polysulfides (LiPSs), 1,3-dioxolane, 1,2-dimethoxyethane and tetrahydrofuran ether-based solvents have been studied using first-principles calculations. It has been found that the (micropore-scale) interlayer of bi-C3N4 shows intimate contact and strong binding with S8 and LiPSs due to the formation of chemical Li–N bonds. The incorporation of soluble LiPSs by the wrinkled layers of bi-C3N4 with 5.5–7.2 Å interlayer pores can suppress the shuttling effect. The interlayer ultramicropores with interlayer distances of <4 Å can accommodate the small Li2S2 and Li2S molecules, and impede the irreversible reaction between the solvents and the LiPSs. The calculated energy gap of bi-C3N4 decreases to be narrow during lithiation. Our results can provide a guideline for promoting the electrochemical performance of microporous g-C3N4/sulfur composites for Li–S batteries.


 Please wait while we load your content...
Please wait while we load your content...