Ultrafast dynamics of UV-excited trans- and cis-ferulic acid in aqueous solutions†
Abstract
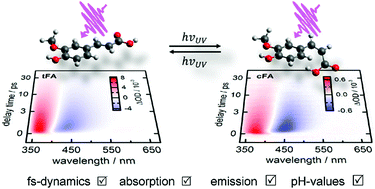
The ultrafast UV-induced processes of the neutral, anionic and dianionic forms of trans- and cis-ferulic acid (FA) in aqueous solution were studied by static and femtosecond time-resolved emission and absorption spectroscopy combined with quantum chemical calculations. In all cases, initial excitation populates the first 1ππ* state. For the dianionic cis-isomer cFA2−, electronic deactivation takes place with a time constant of only 1.4 ps, whereas in all other cases, excited-state deactivation happens more than ten times slower, on a time scale of ≈20 ps. The data suggest sequential de-excitation pathways, where initial sub-picosecond solvent rearrangement and structural changes are followed by internal conversion to an intermediate excited electronic state from which deactivation to the ground state proceeds. Considering the time scales, barrierless excited-state pathways are suggested only in the case of cFA2−, where the observed formation of the isomerisation photoproduct tFA2− provides clear evidence for a cis ⇄ trans isomerisation coordinate. In the other cases, pathways with an excited-state energy barrier, presumably along the same coordinate, are likely, given the longer excited-state lifetimes.


 Please wait while we load your content...
Please wait while we load your content...