Prediction of superconducting ternary hydride MgGeH6: from divergent high-pressure formation routes†
Abstract
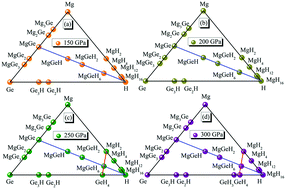
Invigorated by the high temperature superconductivity in some binary hydrogen-dominated compounds, we systematically explored high-pressure phase diagrams and superconductivity of a ternary Mg–Ge–H system using ab initio methods. Stoichiometric MgGeH6 with high hydrogen content exhibiting Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) symmetry was predicted from a series of high-pressure synthesis paths. We performed an in-depth study on three distinct formation routes to MgGeH6, i.e., Mg + Ge + 3H2 → MgGeH6, MgGe + 3H2 → MgGeH6 and MgH2 + GeH4 → MgGeH6 at high pressures. By directly squeezing three elemental solids Mg + Ge + 3H2, we obtained ternary MgGeH6 at 200 GPa. By adding a little bit of the MgGe alloy into hydrogen, we found that MgGeH6 can form and stabilize at about 200 GPa. More intriguingly, upon compressing MgH2 and GeH4 to 250 GPa, we also predicted the same MgGeH6. Electron structure calculations reveal that the cubic MgGeH6 is a good metal and takes on ionic character. Electron–phonon coupling calculation reveals a large λ = 1.16 for MgGeH6 at 200 GPa. In particular, we found that ternary MgGeH6 could be a potential high temperature superconductor with a superconducting transition temperature Tc of ∼67 K at 200 GPa.
symmetry was predicted from a series of high-pressure synthesis paths. We performed an in-depth study on three distinct formation routes to MgGeH6, i.e., Mg + Ge + 3H2 → MgGeH6, MgGe + 3H2 → MgGeH6 and MgH2 + GeH4 → MgGeH6 at high pressures. By directly squeezing three elemental solids Mg + Ge + 3H2, we obtained ternary MgGeH6 at 200 GPa. By adding a little bit of the MgGe alloy into hydrogen, we found that MgGeH6 can form and stabilize at about 200 GPa. More intriguingly, upon compressing MgH2 and GeH4 to 250 GPa, we also predicted the same MgGeH6. Electron structure calculations reveal that the cubic MgGeH6 is a good metal and takes on ionic character. Electron–phonon coupling calculation reveals a large λ = 1.16 for MgGeH6 at 200 GPa. In particular, we found that ternary MgGeH6 could be a potential high temperature superconductor with a superconducting transition temperature Tc of ∼67 K at 200 GPa.


 Please wait while we load your content...
Please wait while we load your content...