Enantioselective synthesis of sulfoxide using an SBA-15 supported vanadia catalyst: a computational elucidation using a QM/MM approach†
Abstract
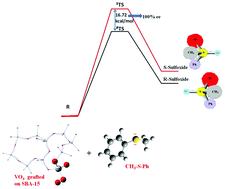
Metal catalyzed asymmetric oxidation of prochiral sulfides is one of the prevailing strategies to produce enantiopure sulfoxides. Keeping in view the reported reactivity of peroxo vanadium complexes towards asymmetric oxidation reactions, this study explores the reactivity of vanadia represented as a VO4 cluster with CH3-S-Ph through DFT computations. The mechanism of the oxidation of sulfides to sulfoxides with unsupported VO4 is thoroughly investigated. The chiral centre in the VO4 cluster is introduced by grafting it on an SBA-15 support and two conformers of the supported cluster are thus obtained. The study was extended to locate transition states for the reaction of each conformer with CH3-S-Ph. The large enantiomeric excess obtained from the energy difference of the transition states confirms the formation of enantiopure sulfoxide. Analysis of the computational results provides a rational explanation for the observed enantioselectivity, which is remarkable. The optical stability as well as asymmetry of chiral sulfoxides obtained by the current approach has been further confirmed by locating the planar transition state, through which conversion from one enantiomer to another takes place. The calculations suggest that transition between the two enantiomers of sulfoxide is hampered by sufficiently high inversion barriers.

 Please wait while we load your content...
Please wait while we load your content...