The role of Tat peptide self-aggregation in membrane pore stabilization: insights from a computational study†
Abstract
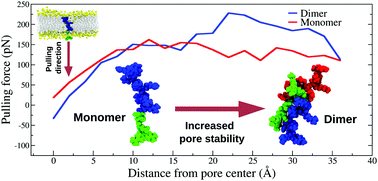
It is widely accepted that endocytosis mediates the uptake of cationic cell penetrating peptides (CPPs) at relatively low concentrations (i.e. nano- to micromolar), while direct transduction across the plasma membrane comes into play at higher concentrations (i.e. micro- to millimolar). This latter process appears to depend on peptide-driven cellular processes, which in turn may induce local perturbations of plasma-membrane composition and/or integrity, and to be favored by peptide aggregation, especially into dimers. Besides, in most studies CPPs are tethered to fluorescent dyes in order to track peptide transduction events under the microscope, although often overlooking the possible role played by the dyes in assisting translocation. In an effort to provide some insights into the transduction process, here we report on a molecular dynamics (MD) simulation study of a prototype of the CPP family, namely the Tat11 arginine-rich motif. To be specific, the translocation of Tat11 across a purposely-created membrane pore, either or not covalently-linked to the tetramethylrhodamine-5-maleimide (TAMRA) dye and in both its monomeric and dimeric form, is analyzed in some detail. Results from several unconstrained and steered MD simulations, as well as energy decomposition analysis, nicely support the latest experimental evidence and help to shed light on key factors enabling peptide transduction. In particular, our study highlights the much slower translocation kinetics of Tat11 dimer in comparison to the single peptide, and therefore its enhanced capability to stabilize membrane pores. Notably, it also shows how TAMRA has overall negligible kinetic and energetic effects on peptide transduction, yet it promotes this process indirectly by favoring peptide aggregation.


 Please wait while we load your content...
Please wait while we load your content...