Hydrotropy and scattering: pre-ouzo as an extended near-spinodal region
Abstract
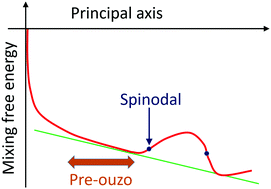
Solubilization or mixing in the presence of hydrotropes is often accompanied by the increase of scattering intensity. When the scattering corresponding to mesoscale structuring grows, the mixed state is called the “pre-ouzo” aggregate, which appears often and is distinct from critical density fluctuation, yet its precise mechanism of appearance is still obscure. Combining the results from the theories of scattering and thermodynamic phase stability with differential geometry, we have constructed a theory which can account for hydrotrope mixing thermodynamics and the pre-ouzo effect in a unified manner. In addition, another well-known signature of hydrotropy, the minimum hydrotrope concentration (MHC) at which solubility of sparingly soluble hydrophobic solutes suddenly increases, has also been linked to the scattering increase. The thermodynamic signatures of the pre-ouzo effect and MHC reveal a mechanistic difference between them, which manifests in the thermodynamic order of derivatives.


 Please wait while we load your content...
Please wait while we load your content...