Fast and accurate prediction of proton affinities: revisiting the extended Koopmans' theorem for protons†
Abstract
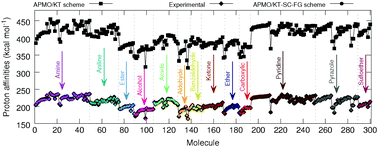
In this work we propose schemes based on the extended Koopmans' theorem for quantum nuclei (eKT), in the framework of the any particle molecular orbital approach (APMO/KT), for the quantitative prediction of gas phase proton affinities (PAs). The performance of these schemes has been tested on a set of 300 organic molecules containing diverse functional groups. The APMO/KT scheme scaled by functional group (APMO/KT-SC-FG) displays an overall mean absolute error of 1.1 kcal mol−1 with respect to experimental data. Its performance in PA calculations is similar to that of post-Hartree–Fock composite methods or that of the APMO second order proton propagator (APMO/PP2) approach. The APMO/KT-SC-FG scheme is also employed to predict PAs of polyfunctional molecules such as the Nerve Agent VX and the 20 common α-amino acids, finding excellent agreement with available theoretical and/or experimental data. The accuracy of the predictions demonstrates that the APMO/KT-SC-FG scheme is a low-cost alternative to adiabatic methods for the calculation of accurate PAs. One of the most appealing features of the APMO/KT-SC-FG scheme, is that PAs can be derived from one single-point APMO Hartree–Fock calculation.


 Please wait while we load your content...
Please wait while we load your content...