Mechanistic insight into selective catalytic combustion of HCN over Cu-BEA: influence of different active center structures†
Abstract
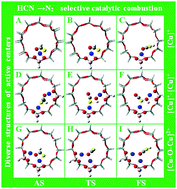
HCN being a highly toxic N-containing volatile organic compound (VOCs) poses great threat to human living environment. Selective catalytic combustion of HCN (HCN-SCC) over metal modified zeolite catalysts has attracted great attention due to related high efficiency and excellent N2 selectivity. In the present work, three types of 24T-Cu-BEA models with different active centers of single [Cu]+, double [Cu]+, and [Cu–O–Cu]2+ were constructed for HCN-SCC mechanism simulations based on density functional theory (DFT). DFT simulation results revealed that HCN-SCC followed an oxidation mechanism over double [Cu]+ through an intermediate of NCO, wherein the synergistic effects of double [Cu]+ active centers were clearly observed, resulting in a significantly lowered energy barrier (1.6 kcal mol−1) during HCN oxidation into NCO. However, an oxidation mechanism (HCN oxidized into NH radical and CO2 through intermediate of HNCO) combining with a hydrolysis mechanism (NH radical hydrolyses into NH3) occurred over single [Cu]+ and [Cu–O–Cu]2+, wherein the NH2 hydrolysis to NH3 step was regarded as the rate determining step with an energy barrier of 72.3 and 74.3 kcal mol−1, respectively. Finally, Mulliken charge transfer (CT) analysis was conducted, based on which the electric properties of different active centers were well illustrated.


 Please wait while we load your content...
Please wait while we load your content...