Transmutation effects on long-term Cs retention in phyllosilicate minerals from first principles†
Abstract
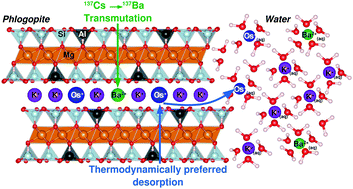
The accidental release and incorporation of radiocesium into soil minerals represents a massive environmental, technical and social challenge. Accurately forecasting the evolving distribution and fate of long- and medium-lived isotopes such as 137Cs and 134Cs over decadal time scales is essential. The cesium cation has long been modeled as a strongly and selectively sorbed species into clay mineral interlayers; however, because of the time scales involved by the radioisotopes half-lives, the effects of radioactive decay on Cs retention have been unknown. We report density functional theory (DFT) simulations of transmutation effects of radiocesium on long-term Cs retention in phlogopite. The calculations show that the progressive appearance of daughter product Ba2+ is accompanied by a proportional increase in thermodynamic driving force to preferentially discharge remaining Cs, both radioactive and stable, back into aqueous solution. Based on thermodynamic analysis, the findings indicate that radiocesium transmutation provides a mean to weaken the binding of Cs in phyllosilicate minerals, therefore potentially involving a premature re-release of Cs back into the environment. In the case where radiogenic Ba2+ ions accumulate in the mineral, collateral effects would ultimately be an increase in the overall interlayer binding energy and a lower resorption capacity.


 Please wait while we load your content...
Please wait while we load your content...