Geometrical flexibility of platinum nanoclusters: impacts on catalytic decomposition of ethylene glycol†
Abstract
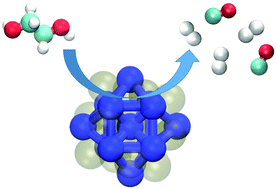
Catalytic decomposition of ethylene glycol on the Pt13 cluster was studied as a model system for hydrogen production from a lignocellulosic material. Ethylene glycol was chosen as a starting material because of two reasons, it is the smallest oxygenate with a 1 : 1 carbon to oxygen ratio and it contains the C–H, O–H, C–C, and C–O bonds also present in biomass. Density functional theory calculations were employed for predictions of reaction pathways for C–H, O–H, C–C and C–O cleavages, and Brønsted–Evans–Polanyi relationships were established between the final state and the transition state for all mechanisms. The results show that Pt13 catalyzes the cleavage reactions of ethylene glycol more favourably than a Pt surface. The flexibility of Pt13 clusters during the reactions is the key factor in reducing the activation barrier. Overall, the results demonstrate that ethylene glycol and thus biomass can be efficiently converted into hydrogen using platinum nanoclusters as catalysts.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...