Ultrafast dynamics of ionic liquids in colloidal dispersion†
Abstract
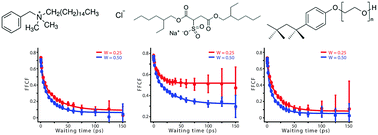
Ionic liquid (IL)–surfactant complexes have significance both in applications and fundamental research, but their underlying dynamics are not well understood. We apply polarization-controlled two-dimensional infrared spectroscopy (2D-IR) to study the dynamics of [BMIM][SCN]/surfactant/solvent model systems. We examine the effect of the choice of surfactants and solvent, and the IL-to-surfactant ratio (W-value), with a detailed analysis of the orientation and structural dynamics of each system. Different surfactants create very different environments for the entrapped ILs, ranging from a semi-static micro-environment to a fluxional environment that evolves even faster than the bulk IL. The oil-phase also clearly affects the microscopic dynamics. The anisotropy decay for entrapped ILs completes within 10 ps, which is similar to free thiocyanate ion in water, while a significant reorientation-induced spectral diffusion (RISD) effect is observed. The entrapped ionic liquid are highly dynamic for all W-values, and no core–shell structure is observed. We hypothesize that, instead of an ionic liquid-reverse micelle (IL-RM), the microscopic structure of this system is small colloidal dispersions or pairs of IL and surfactants. A detailed analysis of the polarization-controlled 2D-IR spectra of AOT system reveals a potential ion-exchange mechanism.


 Please wait while we load your content...
Please wait while we load your content...