A DFT study on the mechanism of photoselective catalytic reduction of 4-bromobenzaldehyde in different solvents employing an OH-defected TiO2 cluster model†
Abstract
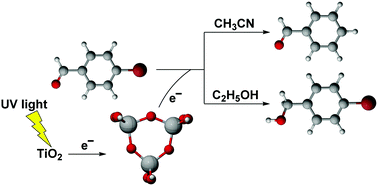
Density functional theory calculations are employed to study the mechanism of photoselective catalytic reduction of 4-bromobenzaldehyde (4-BBA) in acetonitrile and in ethanol solvents. A totally relaxed Ti3O9H6 cluster model is proposed to represent titanium dioxide (TiO2) surfaces. The reduction selectivity of an adsorbed 4-BBA molecule on Ti3O9H6 has been investigated. Owing to the difference in the proton and H atom donating capabilities between explicit CH3CN and C2H5OH solvent molecules, the photocatalytic reduction of 4-BBA is the debromination process in acetonitrile, whereas in ethanol it is the carbonyl reduction process. Therefore 4-BBA can be selectively reduced to benzaldehyde in acetonitrile and 4-bromobenzyl alcohol in ethanol, respectively. Our computational results have verified the reaction mechanism proposed by experiments and show that the debromination of 4-BBA would be efficient if both 4-BBA and Ti3O9H6 have an extra photoelectron. The Ti3O9H6 cluster, playing a role as a hydrogen source and a bridge to transfer photoelectrons from bulk TiO2, would have potential to be an ideal molecular model for understanding photocatalytic reactions on the TiO2 surface.


 Please wait while we load your content...
Please wait while we load your content...