Evidence for cooperative Na+ and Cl− binding by strongly hydrated l-proline†
Abstract
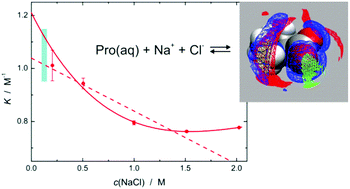
In nature the amino acid L-proline (Pro) is a ubiquitous and highly effective osmolyte protecting cells against osmotic stress. To understand this effect knowledge of the hydration of Pro and its interactions with dissolved salts is essential. We studied these properties by combining statistical mechanics and broadband dielectric spectroscopy and found that Pro remains strongly hydrated up to high amino-acid concentrations. This is also the case upon NaCl addition to a 0.6 M Pro solution. Here, additionally a Pro·NaCl aggregate is formed with a stability constant of K° ≈ 0.95…1.25 M−1, where Na+ and Cl− cooperatively bind to adjacent carboxylate-oxygen and ammonium-hydrogen atoms, respectively.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...