Noncovalent interactions underlying binary mixtures of amino acid based ionic liquids: insights from theory†
Abstract
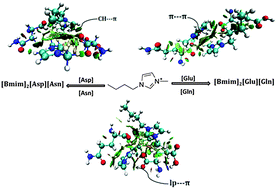
Mixtures of ionic liquids formed by blending a common 1-methyl-3-butylimidazolium [Bmim] cation with the dicarboxylic amino acid anions viz., aspartic acid [Asp], asparagine [Asn], glutamic acid [Glu], and glutamine [Gln], have been investigated by employing dispersion corrected density functional theory. Binary mixtures of [Bmim]2[Asp][Asn] and [Bmim]2[Glu][Gln] ionic liquids emerge with distinct structural patterns. Competition between the constituting anions towards cationic binding sites in acidic and basic (polar) amino acid binary mixtures engenders diverse noncovalent interactions, viz., C–H⋯O hydrogen bonding, π–π stacking, and lp⋯π and CH⋯π interactions, which impart local liquid structure to these systems governing the structural and physicochemical properties of such double salt ionic liquids (DSILs). The DSIL conformers reveal distinct structural features arising from the middle, normal and front arrangements of anions combined with parallel, antiparallel, rotated or displaced orientations of the cations. The inclusion of dispersion corrections through the D3 method affects their binding energies significantly bringing forth alteration in their energy rank order. Molecular insights accompanying the ion aggregates provide directives for the use of DSILs with improved performance in tribological applications.


 Please wait while we load your content...
Please wait while we load your content...