Self-aggregation propensity of the Tat peptide revealed by UV-Vis, NMR and MD analyses†
Abstract
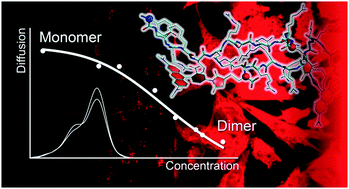
By a combination of UV-Vis analyses, NMR-based diffusion measurements and MD simulations we have demonstrated for the first time that the HIV-1 Tat arginine-rich peptide (Tat11) is able to self-aggregate in both its fluorescently labeled and unlabeled variants. We propose Tat11 dimerization as the dominant aggregation process and show that the associated equilibrium constant increases ten-fold by labeling with the standard TAMRA dye. Also, we extend similar conclusions to other cationic cell penetrating peptides (CPPs), such as Antennapedia (Ant) and nona-arginine (R9).


 Please wait while we load your content...
Please wait while we load your content...