The influence of water content in a proton-conducting ionic liquid on the double layer properties of the Pt/PIL interface†
Abstract
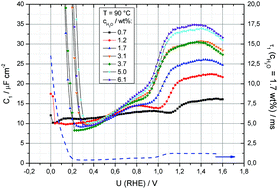
The influence of the water content of 2-sulfoethylmethylammonium trifluoromethanesulfonate [2-Sema][TfO] on the double layer properties of the interface of platinum and the proton conducting ionic liquid (PIL) is investigated by means of impedance spectroscopy and cyclic voltammetry. By fitting the impedance spectra as complex capacitances, up to four differential double layer capacitances and corresponding time constants are obtained, depending on the potential (U = 0–1.6 V/RHE), water content (0.7–6.1 wt%) and temperature (T = 70–110 °C). Within the whole potential range investigated, a high frequency capacitance, C1, and a low frequency capacitance, C2, can be calculated. In the potential region of hydrogen underpotential deposition (HUPD), C1 can be separated into two parts, C1a and C1b. Whereas the high frequency capacitive processes can mainly be attributed to ion transport processes in the double layer, the low frequency process is ascribed to changes in the interfacial layer, including ad-/desorption and Faradaic processes. Alternative interpretations regarding the reorientation of ions, reconstruction of the metal surface and partial electron transfer between anions and Pt are considered.


 Please wait while we load your content...
Please wait while we load your content...