The role of O(1D) in the oxidation mechanism of ethylene by iodosobenzene and other hypervalent molecules
Abstract
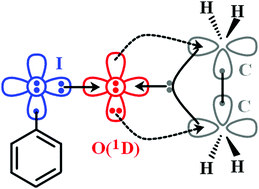
The role of the first excited state of oxygen (1D) is proven essential for the description of terminal iodine–oxygen chemical bonds. The description of the I–O bond as a dative one from iodine to O(1D) provides a simple and accurate picture which explains the oxidation properties of iodosobenzene and similar in nature molecules.


 Please wait while we load your content...
Please wait while we load your content...