The exemplary role of nanoconfinement in the proton transfer from acids to ammonia†
Abstract
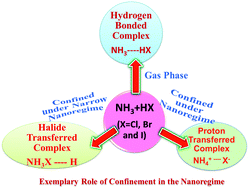
Proton transfer processes from mineral acids to bases (HX, where X = F, Cl, Br and I to ammonia) are normally feasible in solution and they cannot spontaneously occur in the gas phase. We demonstrate that this process can be feasible under nanoconfinement without using any solvent molecules. More interestingly, in contrast to the general observation, halide ions except fluoride behave like protons under high confinement, leading to the formation of NH3X instead of NH4 ions. The triggering transformation of hydrogen bonded to the proton transferred complex under nanoconfinement is explained based on the thermodynamic quantity, static pressure.


 Please wait while we load your content...
Please wait while we load your content...