A simple bulk modulus model for crystal materials based on the bond valence model†
Abstract
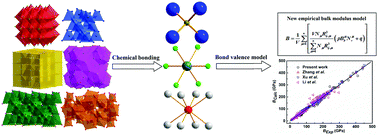
An empirical model based on the bond valence model has been presented to predict the bulk modulus of crystal materials. The bond bulk modulus, in terms of bond valences and bond length, is introduced to describe the resisting ability of a chemical bond to compression. Both the bond bulk modulus and bond density are proposed to be the most important parameters related to the bulk modulus of crystals. For typical ANB8−N and AmBn type crystals, the calculated bulk modulus is in good agreement with experimental values. For multibond crystal systems, the bulk modulus can be equivalent to an average of the bulk modulus of all constituted binary systems. Applied to spinel, B–C–N, polymorphic AR2O4, and chalcopyrite type multibond compounds, their calculated bulk moduli agree well with the available experimental results. Our bulk modulus model can offer a simple and reliable prediction based on the well-described nature of chemical bonding, which makes it powerful for extensively exploring novel superhard materials with low compressibility, and for interpreting geophysical problems.


 Please wait while we load your content...
Please wait while we load your content...