Low temperature conductivity and ion dynamics in silver iodide–silver metaphosphate glasses
Abstract
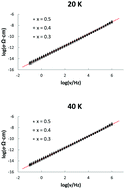
Silver iodide–silver metaphosphate glasses xAgI·(1 − x)AgPO3 (x = 0.3, 0.4, and 0.5) have been prepared using the usual melt quenching method. Differential scanning calorimetry has been used to determine the glass transition temperature of the samples. Impedance spectroscopy spanning wide temperature (20 K to 200 K) and frequency (10−1 Hz to 106 Hz) ranges has been employed to investigate the ion dynamics. At high temperatures, below the glass transition temperature and down to around 120 K, the dynamics show the usual behavior of dc and dispersed conductivity due to the random and correlated motion of the ions. The dc conductivity of the glasses varies dramatically and it increases with the AgI content as expected. At the lowest temperatures investigated, however, the conductivity of the glasses was indistinguishable. Hence, the low temperature dynamics are identical irrespective of the amount of AgI and the structure of the glass. In addition, a nearly constant loss behavior, independent of the temperature and composition, was attained at the lowest temperatures.


 Please wait while we load your content...
Please wait while we load your content...