Photoinduced dimerization of a photosensory DNA-binding protein EL222 and its LOV domain†
Abstract
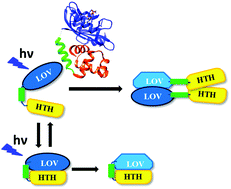
EL222 is a blue light sensor protein consisting of a light-oxygen-voltage (LOV) domain (EL-LOV domain) at the N-terminus and a helix-turn-helix DNA-binding domain at the C-terminus. EL222 acts as a light dependent transcriptional factor. The photochemical reactions of EL222 and the light sensing properties of the LOV domain were investigated. Concentration dependent experiments revealed that the EL-LOV domain is in equilibrium between the dimer and the monomer in the dark state, and the main photoreaction is the dimerization reaction between a monomer in the ground state and that in the excited state. The equilibrium constant and the intrinsic rate constants of dimerization were determined. EL222 was found to also exhibit photoinduced dimerization even in the absence of target DNA, although the yield of the reaction was low (∼0.08 compared with that of the EL-LOV domain). This observation suggests that there are inhomogeneous conformations, open and closed types, of EL222 in solution.


 Please wait while we load your content...
Please wait while we load your content...