Styryl dye formation promoted by catalytic centers of piperazine bound to a silica surface traced by single molecule fluorescence microscopy†
Abstract
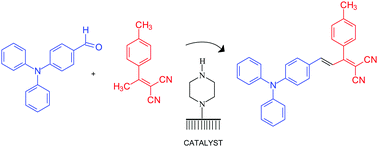
The styryl dye (E)-2-[3-[4-(diphenylamine) phenyl]-1-(p-tolyl)-allylidene]-malononitrile (DFTAM) was prepared by Knoevenagel condensation using homogeneous and surface bound amino catalysts. The catalysis by surface bound piperazine allowed the study of the condensation reaction at a single molecule (SM) level using total internal fluorescence microscopy (TIRFM). The turnover rate of the surface catalyst is dependent on the presence of the byproduct water of the condensation reaction. The addition of zeolite particles as water traps improves the catalytic activity indicated by the highest number of emissive centers mapped with super resolved fluorescence imaging and longest intensity–time trajectories showing SM bursting events. This particular condensation reaction highlights the applicability of the method to study multi-step organic reactions in the condensed phase in which the product is a fluorescent species.


 Please wait while we load your content...
Please wait while we load your content...