Investigating synergistic interactions of group 4, 5 and 6 metals with gold nanoparticles for the catalysis of the electrochemical hydrogen evolution reaction
Abstract
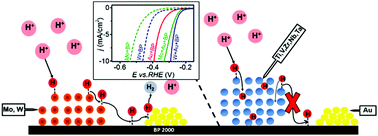
An investigation of the catalysis of the electrochemical hydrogen evolution reaction (HER, 2H+ + 2e− → H2) in aqueous 0.5 M H2SO4 electrolyte using composites consisting of gold nanoparticles (AuNP), carbon (Black Pearl 2000) and group 4, 5, and 6 metals is presented. This study is a continuation of our earlier work (Phys. Chem. Chem. Phys., 2016, 18, 21548–21553) on molybdenum and AuNP, which we found to interact synergistically to enhance the HER. We demonstrate here that tungsten not only also showed synergy with AuNP, but the extent of synergy is even larger than that of the Mo–AuNP composite. The average overpotential needed by the tungsten-based composite catalyst to drive a H2 current density (jH2) of 10 mA cm−2 was 300 mV. In contrast, other metals such as Ti, Zr, V, Nb and Ta did not have any observable synergy with AuNP. Our experimental results indicate that the absence of synergy with these non-performing composites could be related to the absorption of hydrogen into the bulk lattice of the metals to give hydrides. The strong binding of H to these metals could have also prevented their further reaction. We also propose that the aforementioned metal hydrides suppressed HER because they are ineffective for the initial proton discharge step.


 Please wait while we load your content...
Please wait while we load your content...