Potential energy surface stationary points and dynamics of the F− + CH3I double inversion mechanism†
Abstract
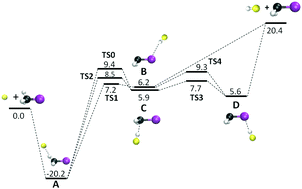
Direct dynamics simulations were performed to study the SN2 double inversion mechanism SN2-DI, with retention of configuration, for the F− + CH3I reaction. Previous simulations identified a transition state (TS) structure, i.e. TS0, for the SN2-DI mechanism, including a reaction path. However, intrinsic reaction coordinate (IRC) calculations from TS0 show it is a proton transfer (PT) TS connected to the F−⋯HCH2I SN2 pre-reaction complex and the FH⋯CH2I− proton transfer post-reaction complex. Inclusion of TS0 in the SN2-DI mechanism would thus involve non-IRC atomistic dynamics. Indeed, trajectories initiated at TS0, with random ensembles of energies as assumed by RRKM theory, preferentially form the SN2-DI products and ∼70% follow the proposed SN2-DI pathway from TS0 to the products. In addition, the Sudden Vector Projection (SVP) method was used to identify which CH3I vibrational mode excitations promote access to TS0 and the SN2-DI mechanism. Results of F− + CH3I simulations, with SVP specified mode excitations, are disappointing. With the CH3 deformations of CH3I excited, the SN2 single inversion mechanism is the dominant pathway. If the CH stretch modes are also excited, proton transfer dominates the reaction. SN2-DI occurs, but with a very small probability of ∼1%. The reasons behind these results are discussed.


 Please wait while we load your content...
Please wait while we load your content...