Sequentially surface modified hematite enables lower applied bias photoelectrochemical water splitting†
Abstract
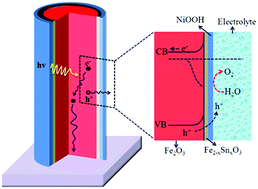
Hematite (α-Fe2O3) is a suitable candidate for photoelectrochemical water splitting due to its well-suited band structure, stability, and availability. However, water splitting using a low external potential is the major challenge that limits the practical application of hematite. Here, we achieve a very low onset potential using a sequential surface treatment approach to overcome two fundamental limiting factors, sluggish hole transfer, and interfacial recombination, independently. First, a heavily doped Fe2−xSnxO3 surface passivation layer was created by Sn4+ surface treatment which can robustly inhibit interfacial recombination. Then, an NiOOH catalyst layer was deposited that greatly enhances the charge transfer process across the passivated electrode/electrolyte interface. By exploiting this approach, the optimized sequentially treated photoanode (Fe2O3/Fe2−xSnxO3/NiOOH) exhibits a low photocurrent onset potential of 0.49 V vs. RHE and a saturated photocurrent density of 2.4 mA cm−2 V at 1.5 V vs. RHE. Transient photocurrent and impedance spectroscopy measurements further reveal that the combined Fe2−xSnxO3/NiOOH layers reduce interfacial recombination and enhance charge transfer across the electrode/electrolyte interface. The results provide convincing evidence that it is possible to address the problems of surface trap recombination and sluggish catalysis independently by employing surface passivation layers first and catalysts later sequentially.


 Please wait while we load your content...
Please wait while we load your content...