A comparative study on the N-heterocyclic carbene adducts of Ih-C60, D5h-C70 and Sc3N@Ih-C80†
Abstract
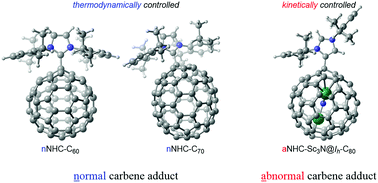
We found that the standard B3LYP and dispersion-corrected B3LYP-D3 methods predicted completely opposite energy order for the Lewis acid–base adducts formed by Ih-C60 or D5h-C70 with normal C2-bound (nNHC) and abnormal C5-bound (aNHC) N-heterocyclic carbenes. By using the validated B3LYP-D3 method and taking the solvent effects into account, the exclusive formations of the nNHC-C60/70 (Li et al. J. Am. Chem. Soc., 2011, 133, 12410–12413) and aNHC-Sc3N@Ih-C80 complexes (Chen et al. Chem. Sci., 2016, 7, 2331–2334) in two recent experiments were suggested to be thermodynamically and kinetically controlled, respectively. In contrast to the reported reactions of endohedral metallofullerenes, aNHC-Sc3N@Ih-C80 hardly isomerizes to the low-energy normal adducts under heat treatment probably due to the substantial energy barrier and excess NHC reagent used in the experiment. Furthermore, the highly regioselective addition of aNHC to the triple-hexagon-junction carbon atom of Sc3N@Ih-C80 was rationalized by using the frontier molecular orbital theory.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...