First principles studies on the selectivity of dimethoxymethane and methyl formate in methanol oxidation over V2O5/TiO2-based catalysts†
Abstract
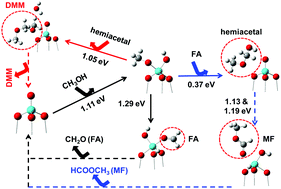
First principles calculations using both molecular cluster and periodic slab models were performed to reveal the mechanism for the formation of dimethoxymethane (DMM) from methanol over V2O5/TiO2-based catalysts. Two different pathways were found, and the formation of DMM was predicted to be initiated by methanol chemisorption followed by a dehydration reaction with hemiacetal catalyzed by acidic sites. For unpromoted V2O5/TiO2 catalysts, we predicted the energy barrier for the rate determining step (RDS) to follow the order formaldehyde (FA) > methyl formate (MF) > DMM, consistent with the experimental observation for the preferential formation of DMM at a relatively low temperature and that of MF at a relatively high temperature. For sulfate-promoted catalysts, the energy barriers were calculated to follow the order FA > DMM > MF, so the sulfate promoter was predicted to mainly enhance the selectivity of MF, consistent with our previous experiment in which very high yield of MF was obtained with the sulfate-promoted catalyst. Calculated rate constants for the RDS were further used for semi-quantitative predictions of the product selectivities, which were found to be in quite good agreement with some of the recent experimental data in the literature, showing the validity of our approach. We also investigated the effects of the titania support and the polymerization of the vanadia species on the reactivity of the V2O5/TiO2 catalyst. Finally, we benchmarked several popular exchange–correlation functionals for calculating the reaction energies for the formation of FA, MF, and DMM from methanol oxidation, and the M06 hybrid functional was found to be superior to other semi-local and hybrid functionals studied.


 Please wait while we load your content...
Please wait while we load your content...