Monitoring aggregation of a pH-responsive polymer via proton exchange†
Abstract
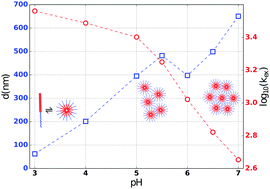
Understanding the changes in the macro-structure of amphiphilic pH-responsive polymers remains a relevant issue due to their potential use as drug delivery carriers. Since some of the amphiphilic polymers are known to exchange hydrogen ions with an aqueous solvent, we monitor the effective change of the surface to volume ratio of such polymer aggregates using solution-state nuclear magnetic resonance (NMR) spectroscopy. The surface to volume ratio with the help of UV-visible spectroscopy is shown to yield the average diameter of the polymer aggregates. We show that the proposed method not only satisfactorily corroborates the existing notions of how the aggregation of these polymers takes place as a function of pH, but also provides a quantitative estimate of the size of the aggregates.


 Please wait while we load your content...
Please wait while we load your content...