Comparison of high pressure and nanoscale confinement effects on crystallization of the molecular glass-forming liquid, dimethyl phthalate
Abstract
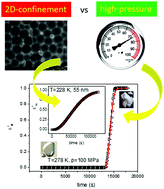
High pressure and nanoscopic confinement are two different strategies commonly employed to modify the physicochemical properties of various materials. Both strategies act mostly by changing the molecular packing. In this work, we performed a comparative study on the effect of compression and confined geometry on crystallization of a molecular liquid. Dielectric spectroscopy was employed to investigate the crystallization of the van der Waals liquid, dimethyl phthalate, in nanoporous alumina of different pore sizes as well as on increased pressure (up to 200 MPa). The analysis of the crystallization kinetics under varying thermodynamic conditions revealed that both strategies affect the crystallization behavior of the sample in very distinct ways. Compression shifts the maximum crystallization rate towards a higher temperature and broadens it. As a result, it is more challenging to avoid crystallization upon cooling the liquid at high pressure. In contrast, when the same material is incorporated into nanopores, crystallization significantly slows down and the maximum rate shifts towards a lower temperature with decreasing pore size. Finally, we show that crystallization in nanoporous alumina is accompanied by pre-crystallization effects upon which a shift of the α-relaxation peak is observed. An equilibration process prior to the initiation of crystallization was detected for the confined material both above and below the glass transition temperature of the interfacial layer, while not in the bulk.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...